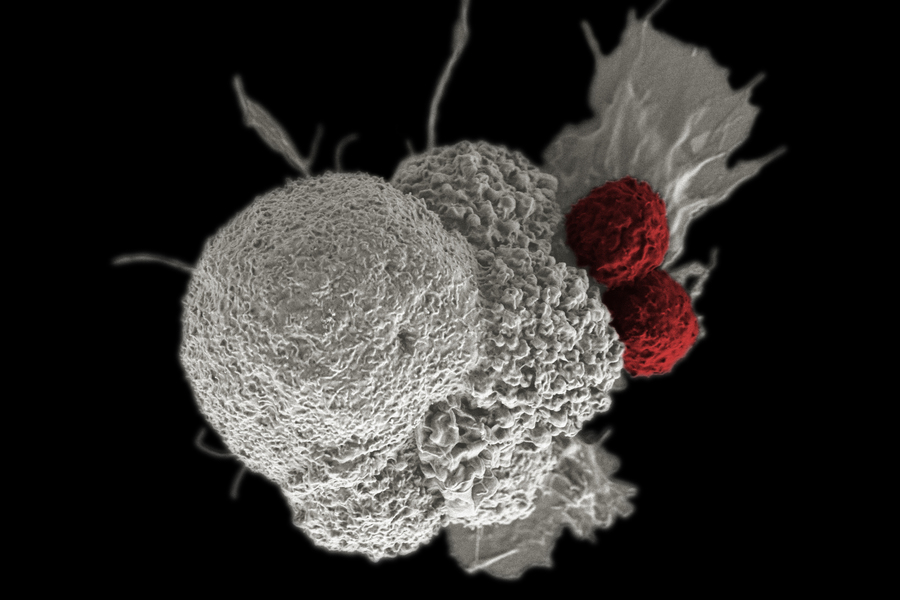
If you’ve ever wished a cell could follow instructions like a tiny, polite robot—“sense this, then do that”—synthetic biologists have good news. In 2025, researchers reported a modular construction kit for building sense-and-respond circuits inside human cells, moving the field from clever demos to fast, programmable systems that could one day help treat cancer, autoimmune flares, and more.
What “Living Circuits” Actually Are
Engineered gene circuits let cells process inputs (like a disease signal) and trigger outputs (like releasing a protein or switching on a gene). Historically, many circuits relied on transcription—turning genes on/off—which is powerful but slow (often hours). A 2025 Rice University study instead assembled circuits out of phosphorylation cycles—the same fast protein-to-protein signaling cells already use—so responses happen on the order of seconds to minutes. In other words, less hourglass, more lightning.
“Imagine tiny processors inside cells made of proteins that can ‘decide’ how to respond to specific signals like inflammation or tumor markers.” — Rice University news release summarizing the 2025 Science paper (Yang et al.), which introduced a kit for engineering synthetic phosphorylation circuits in human cells.
Visual Cheat-Sheet: Two Ways to Wire a Cell
| Feature | Transcriptional Circuits | Phosphorylation-Based Circuits |
| Main parts | DNA → RNA → protein (gene expression control) | Protein–protein signaling with phosphate “tags” |
| Typical speed | Often hours to see full effect | Seconds to minutes |
| Strengths | Deep programmability, durable memory | Rapid, tunable responses; can amplify weak signals |
| 2025 highlight | Longstanding work in bacteria and mammalian cells | Rice team’s modular kit in human cells (peer-reviewed in Science) |

Timing and amplification claims are based on the Rice study’s summary and results overview.
Why Scientists Care (and Why You Might, Too)
- Faster feedback for living medicines
Cell therapies that fight tumors need to tell friend from foe in real time. MIT researchers highlight logic-gated immune cells—circuits that act like digital AND/NOT gates—to attack cancer while sparing healthy tissue. Early clinical signals from Senti Bio’s logic-gated approach in blood cancers suggest this strategy is feasible. - On-site detection with whole-cell biosensors
Engineered cells can act as field-ready sensors, converting chemicals or biomarkers into easy-to-read signals via embedded circuits. Reviews in 2025 emphasize their selectivity and low cost for on-site detection, from environmental toxins to clinical markers. - A broader toolbox for “living therapeutics”
The trend goes beyond one lab: 2025 surveys discuss how synthetic biology + AI-aided design are building “living” medicines (engineered cells, smarter circuits, thermostable vaccine concepts). The direction of travel is clear—more programmable biology at human-relevant speed.
How the 2025 Rice Advance Changed the Conversation
The Rice team’s core insight was to treat each phosphorylation cycle as a reusable unit, then link units to build entirely new signaling routes between a chosen input and output. Their synthetic circuits amplified weak inputs and ran in parallel with a cell’s native networks without hurting growth—key for future therapeutics. The work was published in Science (DOI 10.1126/science.adm8485) and showcased as a “major breakthrough” because it pushes mammalian cell circuits toward speed, tunability, and modularity at once.
What’s Ready Now vs. What’s Next
- Now: In research and early clinical contexts, you’ll see logic-gated immune cells (e.g., Senti Bio) and biosensor strains for lab/field detection. These aren’t over-the-counter products; they’re carefully regulated and trialed.
- Next: Faster, on-demand responses to human signals—think detecting inflammatory cytokines or tumor markers and responding immediately—are closer thanks to phosphorylation-based designs, but still need optimization and safety proofs before widespread use.

Plain-Language FAQ
Is this CRISPR?
CRISPR is a genome editor. Circuits are programs running in cells.They can work together, but a circuit can be built without cutting DNA every time; the 2025 kit largely uses engineered proteins and phosphorylation to pass messages quickly.
How is this safer than “always-on” therapies?
Logic gates and fast shutoffs let cells act only when multiple disease signals align and stand down when they don’t—raising the chance of hitting tumors hard without friendly fire. That selectivity is the point of logic-gated designs highlighted by MIT.
Will “smart cells” replace drugs?
More likely they’ll combine with drugs—cells to sense and decide, drugs or proteins as the effectors. Reviews in 2025 frame this as part of broader living therapeutics, not a replacement for all medicines.
One-Minute Summary (for Skimmers)
- In 2025, Rice University unveiled a modular kit for phosphorylation-based circuits in human cells, enabling second-to-minute responses and signal amplification—peer-reviewed in Science.
- Logic-gated immune cells (MIT/Senti Bio) show how circuits make cancer cell therapies more selective, with early human data in AML.
- Whole-cell biosensors use circuits to detect targets on site, offering selective, low-cost diagnostics outside big labs.